Turbidity of water is an optical property that causes light to be scattered and absorbed, rather than transmitted. The scattering of light that passes through a liquid is primarily caused by suspended solids. The higher the turbidity, the greater the amount of scattered light. Even a very pure fluid will scatter light to a certain degree; no solution will have zero turbidity.
There are different measurement standards used based on applications, and with these standards are applied units. The ISO standard adopted the FNU (Formazin Nephelometric Unit) while the EPA uses the NTU (Nephelometric Turbidity Unit). Other units include the JTU (Jackson Turbidity Unit), FTU (Formazin Turbidity Unit), EBC (European Brewery Convention Turbidity Unit) and diatomaceous earth (mg/L SiO₂).
There is a number of application areas where turbidity is a required parameter to measure.
Monitoring for Natural Water Supplies
In natural water, turbidity measurements are taken to gauge general water quality and its compatibility in applications where there are aquatic organisms. It has been found that there is a strong correlation between turbidity and BOD (Biochemical Oxygen Demand) value. Moreover, by definition, turbidity obstructs light, thus reducing the growth of marine plants, eggs and larvae, which are usually found in the lower levels of an aquatic ecosystem.
Historically, turbidity is one of the main parameters monitored in wastewater. In fact, the monitoring and treatment process was once solely based on the control of turbidity. Currently, the measurement of turbidity at the end of the wastewater treatment process is necessary to verify that the values are within regulatory standards.Generally speaking, the turbidity value has to be between 0 and 50 FTU, with an accuracy of ±3 FTU depending on the phase of the wastewater treatment process. By monitoring the turbidity level, it can be determined if the different stages of the process, particularly in the filtration and purification stages, have been completed correctly.
Purification of Drinking Water
Turbidity is one of the most important parameters used to determine the quality of drinking water. Public water suppliers are required to treat their water to remove turbidity. Best practice operation of a conventional water treatment plant should be able to produce treated water with a turbidity of less than 0.1 nephelometric turbidity units (NTU).
According to Indian Indian Drinking Water Guidlines, turbidity cannot be higher than 1.0 nephelometric turbidity units (NTU) at the plant outlet. Adequately treated surface water does not usually present a turbidity problem.
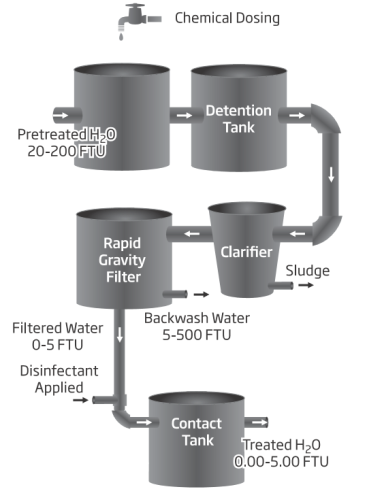
The World Health Organization indicates 5 NTU as the reference turbidity value of water for trade. This value has been established based on the aesthetic characteristics of water. From a hygienic point of view, 1 NTU is the recommended value. Many drinking water utilities strive to achieve levels as low as 0.1 NTU. Turbidity is an indicator and will not give results for a specific pollutant. It will, however, provide information on the degree of overall contamination. The flow chart for the water treatment process of drinking water shows the turbidity reference values for each phase.
Typical sources of turbidity in drinking water include the following:
• Waste discharge
• Run-off from watersheds, especially those that are disturbed or eroding
• Algae or aquatic weeds and products of their breakdown in water reservoirs, rivers, or lakes
• Humic acids and other organic compounds resulting from decay of plants, leaves, etc. in water sources
• High iron concentrations which give water a rust-red coloration (mainly in ground water and ground water under the direct influence of surface water)
• Air bubbles and particles from the treatment process



Comments
Post a Comment